It seems like we finally have the coronavirus drug. It's cheap and widely-available
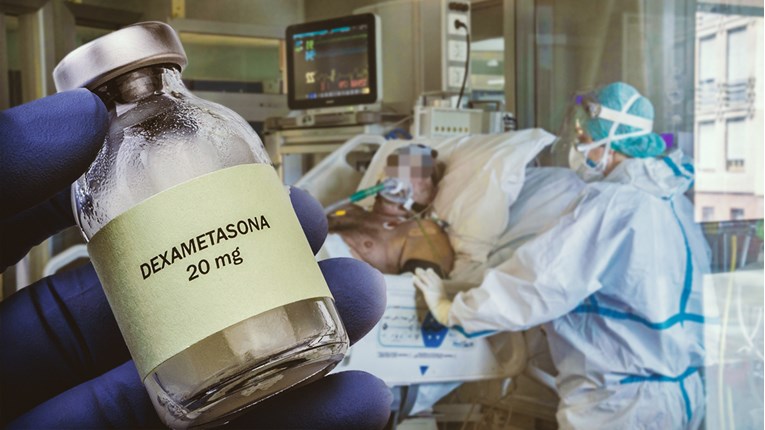
LAST WEEK, the British scientists published that a well-known drug that is widely available and cheap could save many lives.
As Index wrote earlier, the discovery is part of the world's biggest trial of existing treatments called Randomised Evaluation of COVid-19 thERapY (RECOVERY), which studies whether some of the existing drugs can be useful in the coronavirus treatment.
The idea behind this and other similar trials is to speed up finding the treatment by testing drugs that are already approved because of having an effect on some diseases or symptoms similar to COVID-19 and not having dangerous side effects.
Within the trial, besides the anti-malaria hydroxychloroquine and the azithromycin antibiotic (in Croatia known as Summamed), the scientists have also tested some anti-inflammatory drugs because it's known that COVID-19 can cause an overreaction of the immune system, the so-called cytokine storm.
The testing of one of the anti-inflammatory corticosteroids, dexamethasone, showed that in small doses when given to the seriously-ill patients, it can significantly reduce deaths.
The authors have stated for the British media that dexamethasone would have saved around 5,000 lives if it had started to be used for the treatment of patients in the UK from the beginning of the pandemic.
The BBC posted on Twitter that the drug was being widely available across the National Health Service in the UK as of June 17 due to the low prices and fast effect.
Widely-available drug
What is especially interesting in this story is that dexamethasone is widely available and cheap, just like corticosteroids.
Epidemiologists Martin Landray, one of the Chief Investigators in the trial, pointed out that the test results indicate that dexamethasone treatment lasts up to 10 days and costs around £2 per patient for the entire treatment.
He thinks hospitals should start giving dexamethasone to seriously ill patients as soon as possible and warned that it still shouldn't be available without medical prescription.
According to the trial results, dexamethasone doesn't help patients with mild symptoms of COVID-19, i.e., those who don't need oxygen support. It was shown effective only with patients needing oxygen and even more with patients on respirators.
Qualitative research on large sample size and a significant result
This clinical trial included 2,104 patients chosen randomly among hospitalized patients. They were receiving 6mg of the drug (administered orally or intravenously) every day. The other randomly selected group of 4,321 patients received the usual COVID-19 treatment. In this group, the death toll was the highest after 28 days for the patients needing respirators (41%) and somewhat less for patients needing oxygen (25%), and the lowest among patients without any respiratory intervention required (13%).
Dexamethasone reduced the mortality of patients on respirators by one-third and of patients needing oxygen by one-fifth. The patients without respiratory problems didn't benefit from the drug.
Based on these results, the authors concluded that dexamethasone could prevent every eighth death of a patient on a respirator and every twenty-fifth of patients needing oxygen.
"This is, for now, the only drug that has proved to significantly reduce mortality. It's a big step forward," epidemiologist Peter Horby from the University of Oxford commented on the trial results.
Trkulja: It's to be expected that dexamethasone can help
Vladimir Trkulja from the Department of Pharmacology at the Faculty of Medicine said that he had seen the first reports about the drug, and he's not surprised by its effectiveness.
"The acute respiratory distress syndrome and the so-called cytokine storm, as seen in some patients infected with COVID-19, share many pathophysiological similarities with other systemic inflammatory diseases, which are usually treated with dexamethasone, i.e., corticosteroids. If there's no reaction, the treatment is continued with other immunosuppressives as in any other overreaction of the immune system," said Trkulja.
In this case, since it's an infection, it wasn't certain in which moment in the development of the disease will dexamethasone start to be used. The studies showed that since they suppress the immune system, corticosteroids can ease the reproduction of pathogens in an organism in the early phases of the disease. In other words, if corticosteroids are given to patients too soon, the infection can break out more easily.
Large sample size, powerful university, and a solid method
Trkulja thinks that the trial was conducted qualitatively and on large sample size.
"It's a randomized trial, i.e., the trial is based on a randomly chosen sample with good criteria at a good university with good experts. They've asked a very specific question in their trial- will dexamethasone benefit the seriously-ill patients. It's a completely different scenario from the remdesivir treatment, which should suppress the virus reproduction and should be given at the onset of the disease. Remdesivir has slightly shortened the period of disease and slightly reduced mortality. However, no significant effect should have been expected in that case because it was tested in a population that included the patients with mild symptoms, which indicate that the mortality rate is generally not high. On the other hand, dexamethasone is used with seriously ill patients with a high mortality rate (around 40%), so in that patient sample, the effect of the drug is expected to be more expressed," Trkulja explained.
Our pharmacologist said that many doctors in the world and Croatia have been using corticosteroids during the pandemic, including dexamethasone, when they thought that the benefit for a patient would be significantly larger than the possible side effects.
It's also produced in Slovenia
Dexamethasone is usually used to relieve the symptoms of different inflammatory processes. The website of the pharmaceutical company Krka, which also produces this drug, says that it's recommended "as an additional treatment for insufficient secretion of natural corticosteroids in cases of adrenal gland failure," and also "for anti-inflammatory, analgetic, and anti-allergic effect, and reduction of immune system activity."
Besides relieving different inflammatory processes, this drug seems to help stop the cytokine storm, i.e., the overreaction of the immune system during the coronavirus infection, which can be deadly.
The trial results and its methodology are yet to be seen
As much as these results are promising, it should be pointed out that the trial still hasn't been reviewed by independent experts and that it's yet to be published in more serious medical journals. What we have so far is the report of the authors themselves posted on the RECOVERY project's website.
Dragan Lepur, a virologist from the Fran Mihaljevic University Hospital for Infectious Diseases, said he couldn't comment on the trial results because he hadn't seen them yet. "Despite it being a randomized clinical trial with a large number of patients and with alleged powerful therapy effect, only when the trial is published will we know how it was conducted," warned Lepur.
"I think that one should be very careful in this situation. All medical journals are now highly motivated to become the first to publish a trial, even if it's preliminary and not yet reviewed. It all goes very fast because it's urgent and in the interest of public health. But it's a double-edged sword since these trials can have big flaws due to the difficult circumstances in the systems that are falling under the pandemic. It's to be expected that some trials of suspicious qualities will appear. During the last couple of months, we could see for ourselves that big promises weren't kept. Hydroxychloroquine was shown to do more damage than good, and remdesivir has a very moderate impact," the virologist points out.
He thinks that when it comes to clinical trials, one should have in mind that there's still a lot that we don't know about SARS-CoV-2 infection and COVID-19 pathogenesis.
"If we intervene with drugs or procedures in clinical trials with a disease that we don't know enough about, it's easy to get wrong conclusions. Except that we have to know how a certain drug works, we also have to know in what phase it can be administered and what is to expect," he added.
Patients have already been treated with dexamethasone during this pandemic
Patients have already been treated with dexamethasone during this pandemic
Corticosteroids have already been given to seriously-ill patients in the pandemic since it's beginning in China, as well as in other countries. However, despite being administered in significantly larger doses, the results so far have still been inconclusive.
"Administering the drug within an immunomodulatory procedure, i.e., the procedure that modulates the immune system is still a matter of dispute and is quite controversial. We are still not at the disposal of facts that could undoubtedly show that they benefit the patients. They are frequently administered in difficult situations when there's always an emotional component. In that case, the doctors usually say: 'Let's give them medicine, and we'll see what happens because it could help them, and the possibility for harming them is very low.' When you give it to people in uncontrolled conditions, it's difficult to make conclusions," said our interlocutor from Fran Mihaljevic Hospital.
From fear and bans to a meaningful administration of corticosteroids
Immunomodulation has been implemented in infectology for a long time, and corticosteroids are just one of the ways of the immunomodulatory treatment, which were banned at the beginning due to fear that a person could make the microorganism reproduction easier by suppressing the immune system, which could lead to creating more damage than good.
"That's why the immunomodulation, including corticosteroids, has been reserved only for seriously-ill people that didn't have much to lose. But over the years, by getting to know the pathogenesis of infectious diseases, we found out that they are a consequence of the activity of harmful pathogens, bacterias, viruses or parasites, and a consequence of a conflict between invasive microorganisms and immune system response. The problem is that the attempts of an organism to suppress the infection aren't simple. We understand some of its mechanisms, while we don't understand others. Moreover, not every person reacts the same to the same infection."
Corticosteroids have their place in this pandemic
It's considered that corticosteroids make sense in this pandemic, although not necessarily with all patients.
"We've seen that the disease mostly goes through two phases. In the first one, we have an infection with characteristic symptoms, as in most respiratory diseases. In the second phase, the infection mostly persists, but a part of patients develop a fierce reaction of the immune system. The administration of immunomodulatory drugs can be considered in that case. These patients usually end up needing oxygen or respirators. In this epidemic, they've already been treated with corticosteroids. We also had such patients. In these circumstances, a part of the immune reaction can be suppressed. We don't have enough experience with these cases. It probably makes sense to give corticosteroids, but the right timing is important. If given too late, the damage can be irreparable. If given too soon, the infection will spread more easily, especially when we don't have a drug for the virus itself. The problem with COVID-19 is that the patients go to hospitals too late, after ten or more days, when the disease progresses, and it's too late for the effect of a possible antiviral therapy to be clearly visible," Lepur concluded.
.

Brže učitavanje članaka, bez ometanja dok čitate.
10 eura mjesečno.


 Facebook
Facebook
 Google
Google